Designing the Etherena® ESA – Global impact of good design
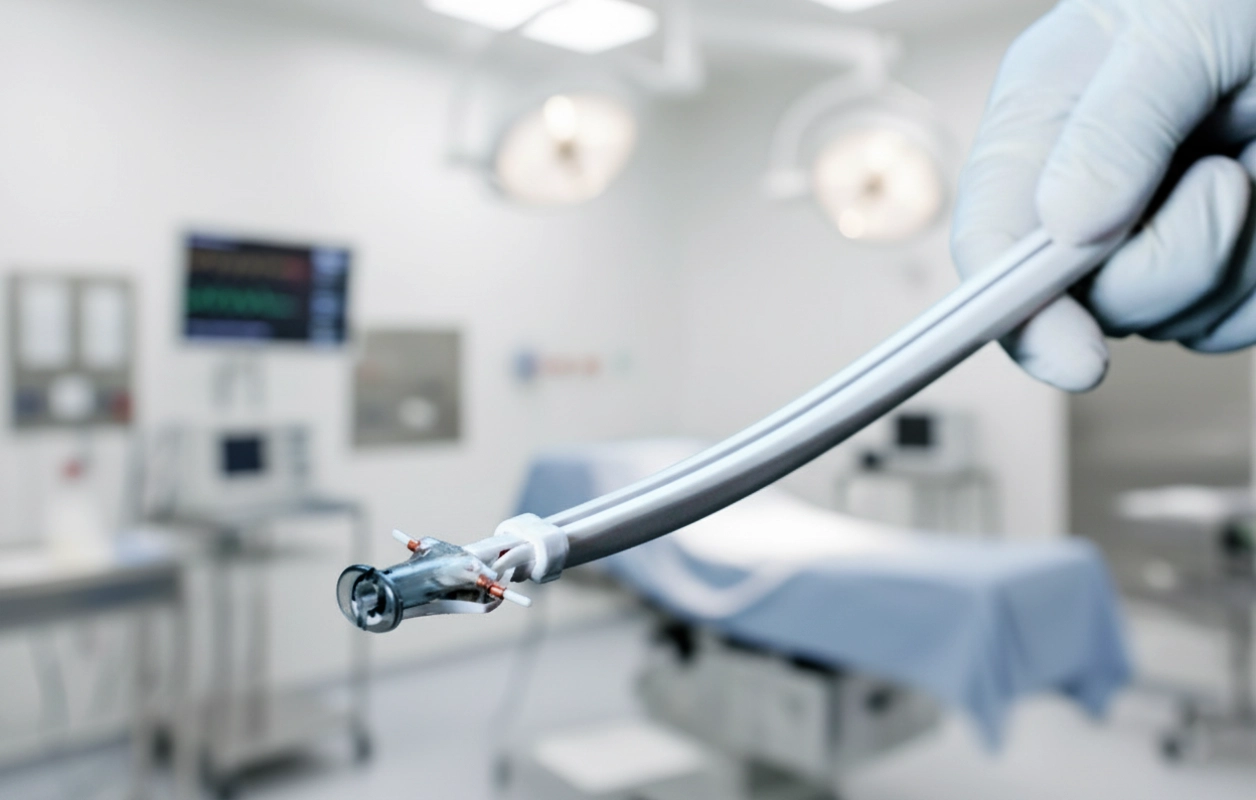
Reimagining a Critical Healthcare Experience
At Ticket Design, we believe thoughtful design can transform healthcare experiences. Our collaboration with Pregna International Ltd., one of the world’s largest contraceptive manufacturers, led to the development of the Etherena® IUD inserter with the ESA uterine sound – a breakthrough device that redefined how intrauterine contraceptives are delivered.
Traditional IUD insertion is often associated with pain, anxiety, and risk of complications such as uterine perforation or expulsion. Our design brief was simple yet ambitious: make IUD insertion safer, more intuitive, and more comfortable – for both healthcare providers and women.
Design Innovations that Matter
- All-in-one system: Etherena integrates the IUD and inserter into a single device, simplifying setup and reducing errors.
- One-handed ergonomics: A push–pull mechanism ensures stability, minimizing provider fatigue during insertion.
- Curved, anatomical design: The inserter follows the natural uterine axis, ensuring accurate placement in both anteverted and retroverted uterus.
- ESA disposable sound: A rounded, serrated-handled uterine sound with depth markers reduces the risk of perforation and offers precise measurement.
- No-touch technique: Designed for aseptic use, Etherena minimizes infection risk.
- Patient comfort: A softer nylon suture and reduced force of insertion improve the overall experience.
Global Reach and Market Presence
Launched in the mid-2010s, Etherena quickly attracted global attention. By 2019, a major distribution partnership with DKT WomanCare Global expanded its availability across Africa, the Middle East, Eastern Europe, and Central Asia. Today, it is part of Pregna’s portfolio sold in 100+ countries.↗
Impact and Measured Success
- Provider Ease-of-Use: In a 2024 clinical trial, 94% of providers found Etherena “easy” to load vs only 26% with the conventional method.
- Improved Accuracy: Placement success rates reached 100% with Etherena, compared to ~86% with standard insertion.
- Patient Comfort: Women reported significantly less pain during sounding with ESA compared to traditional metal sounds (p < 0.0001).
- Reduced Risk: The no-touch technique and depth gauge design lower risks of perforation, infection, and expulsion.
- Industry leaders have praised the device: “Pregna makes high quality products and has invested in pushing the IUD market forward. We are pleased to market their innovative Etherena…” – Rodrigo Portugues, Commercial Director, DKT WomanCare.
Why Design Was the Differentiator
The Etherena project demonstrates the power of human-centered design in medical devices. By deeply analyzing user pain points – from the provider’s grip and fatigue to the patient’s anxiety and pain – the final product went beyond incremental improvement. It reframed the insertion process as a safer, faster, and more reassuring experience.
This is design not as ornamentation, but as a strategic enabler of healthcare outcomes.
References
- Pregna International – Etherena & ESA Technical Brochure
- Medzell Medical Network – Etherena ESA Product Page
- NS Medical News – DKT WomanCare partners with Pregna for Etherena distribution
- DKT WomanCare Global – Product Portfolio
- Cureus Journal – Latika Chawla et al., 2024: Comparative study of Etherena vs conventional insertion (PDF)